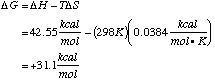Thermodynamics Solutions: #38
38.* (1990 F 13) Consider the reaction CaCO3(s) <–––> CaO(s) + CO2(g) at 1 atm CO2 and 298K. DH° for this reaction is +42.55 kcal/mole, DS° is +38.4 kcal/(mole*K). A. Write an expression for the equilibrium constant for the above stated reaction in terms of DH° and DS°.
B. Calculate the sign and magnitude of DG°.
C. Offer an explanation for why DH° is positive for this reaction. The CaCO3 lattice disruption coupled with the breaking of bonds takes more energy than what is gained from CaO lattice formation and the bonds formed in the product. D. Offer an explanation for why DS° is positive for this reaction. The reaction proceeds from one mole of solid to a mole of solid and a mole of gas. The products are more numerous and more disordered than the reactants. E. Would this reaction or its reverse proceed thermodynamically at 298°K? D G > 0 for the forward reaction, so the reverse reaction is favored at this temperature. (DGrev=-31.1 kcal/mol)F. Would an increase in temperature be expected to promote, retard, or have no effect on the reaction? An increase in temperature would promote the reaction. (It's endothermic and heat would drive the reaction to the right.) G. Would the addition of a catalyst be expected to increase, decrease, or leave unchanged the value of the equilibrium constant? The addition of a catalyst would leave unchanged the value of the equilibrium constant. (Catalysts only affect kinetics.) H. Would the addition of CaCO3 to the reaction mixture be expected to increase, decrease, or leave unchanged the value of the equilibrium constant? The addition of CaCO3 to the reaction mixture would leave unchanged the extent of conversion. (The equilibrium constant depends only on CO2 (g) , so adding CaCO3 does nothing.) |