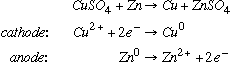Thermodynamics Solutions: #36
36.* (1990 F 2) A. Illustrate with a balanced equation a reaction with negative enthalpy change and a positive entropy change: Some examples: C. Use equations to illustrate a reaction which can be carried out directly or electrochemically:
|