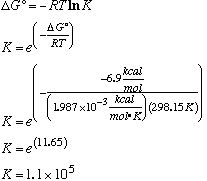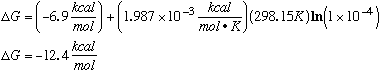Thermodynamics Solutions: #35
35.* (1991 3 6) A. Find the equilibrium constant K for the biologically important hydrolysis reaction: ATP --> ADP + phosphate, which has DG°=6.9 kcal/mol.
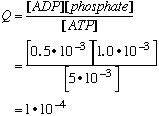
B. Calculate the free energy change for this reaction when [ADP] = 0.5 x 10-3, [phosphate] = 1.0 x 10-3, and [ATP] = 5 x 10-3. These are the concentrations that occur in a typical muscle cell. A catalyst for the hydrolysis reaction is present in the muscle cells.
C. Does the hydrolysis reaction of ATP to ADP reach equilbrium in a muscle cell? How efficient is the catalyst? •From Part B, recall that Q=1.0•10-4 •From Part A, recall that K=1.1•106 •Q << K (The observed reaction quotient is much less (by 9 orders of magnitude) than the equilbrium constant. •The reaction is not at equilibrium in the cell. There must be a large kinetic barrier to reaction even in the presence of the catalyst (enzyme.) •The enzyme catalyst is highly inefficient for the ATP hydrolysis reaction. |