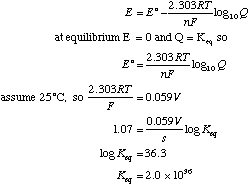Thermodynamics Solutions: #34
34.* (1991 3 2) A. Use the data below to calculate the cell potential E0 for the reaction
B. What is the equilibrium constant for this reaction?
C. Contrary to intuition, dilute solutions of H2O2 are often stable. Offer an explanation for this. There must be a kinetic barrier for the reaction which prevents dilute solutions of H2O2 from decomposing. |