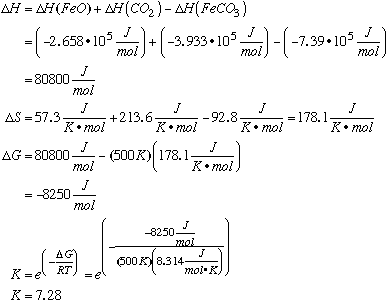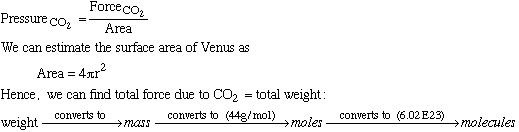Thermodynamics Solutions: #33
33.* (1991 F 13) The CO2 content of the atmosphere of Venus is about 75 atm. Some scientists theorize that this CO2 is produced via the decomposition of siderite and limestone on the planet. A. Write an expression for the equilibrium constant for the decomposition of siderite (FeCO3). FeCO3(s) <--> FeO(s) + CO2(g)
B. Given the values of DH° and DS° in the table below, calculate the equilibrium constant for the decomposition of siderite at 500 K.
C. Write an expression for the equilibrium constant for the decomposition of limestone (CaCO3). CaCO3(s) <--> CaO(s) + CO2(g)
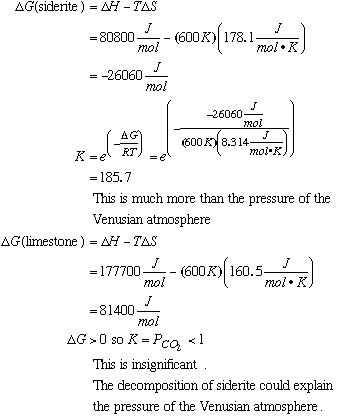
D. Given the values in the table below, compute the equilibrium constant for the decomposition of limestone at 500K.
E. What do your calculations suggest is the partial pressure of CO2 on Venus? Is this in agreement with the measured pressure of 75 atm? Could the decomposition reactions account for the CO2 pressure on Venus? Discuss. At 500K, the partial pressures (equilibrium constants, because the equilibrium constant is the same as the partial pressure as we showed in A and C) of CO2 for each reaction do not total 75 atm. Hence, your initial guess may be that neighbor reaction can explain the pressure of the Venusian atmosphere. However, if the temperature were 600K, calculations for each reaction are as follows:
F. Are aqueous oceans expected to occur on Venus? No. Water boils at 373 K and 1 atm, and even boils at temperatures below 500K at pressures of 50 atm. If you realized that 500K was above the normal boiling point, but was torn as to what effect the pressure would have, you got full credit. :) G. Sketch out a procedure for estimating the number of CO2 molecules in the Venusian atmosphere. At T=500K, we know that
|