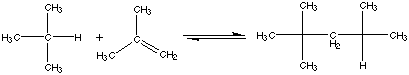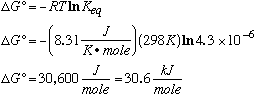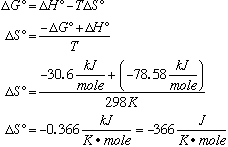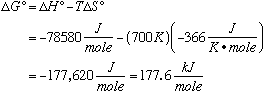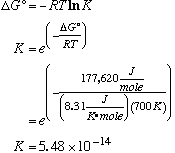Thermodynamics Solutions: #30
30.* (1992 3 1) The following polymerization reaction has DH° = -78.58 kJ/mol at room temperature. The equlilibrium constant is 4.3 x 10-6
A. Is this reaction exothermic or endothermic? At 25°C, DH°=-78.58 kJ/mol. Therefore, the reaction is exothermic (releases heat).B. Calculate DG° at 25°C.
C. Calculate DS° at 25°C.
DS° is negative, signifying that the order of the system is increasing. (2 molecules are becoming 1 molecule.) D. Calculate K at 700°C.
E. Offer an explanation for the different equilibrium constant values at the different temperatures. The forward reaction is less favored at higher temperature because heat is a product of the exothermic forward reaction. If you add heat you drive the reaction toward the reactants (backwards). This is Le Châtelier's Principle! |