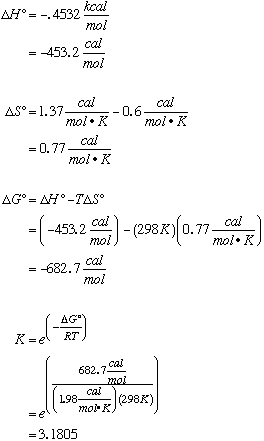Thermodynamics Solutions: #29
29.* (1992 F 17) Consider the reaction diamond --> graphite. DH0 of diamond = 0.4532 kcal/mol and DH0 of graphite = 0 kcal/mol. DS0 of diamond = 0.6 cal/mol*K and DS0 of graphite = 1.37 cal/mol*K. Calculate the equilibrium constant for this reaction at 250C, 298K. As temperature increases, what happens to the equilibrium?
In this case, because DH is negative and DS is positive, as temperature increases, DG increases also. At higher temperatures, we favor the products (graphite). |