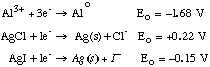Thermodynamics Solutions: #27
27.* (1993 3 1C) C. At the time Stanford was founded, aluminum metal dinner plates were more expensive than solid silver plates whereas today the opposite is true. Explain. Look at Eo… From natural sources, oxides and/or salts, reducing Al to Alo will be more difficult since it is highly thermodynamically unfavorable… Not true for Ag salts. It was difficult to find reducing agent with Eo > +1.68 V until electrolysis was perfected ~1900 A.D. |