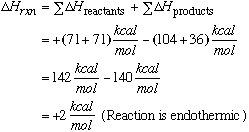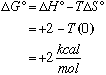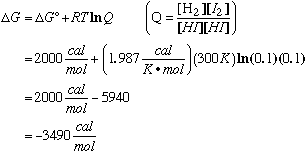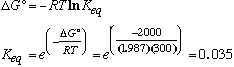Thermodynamics Solutions: #26
26.* (1993 F Part II 1) The hydrogen iodide molecule, HI, has a bond energy of 71 kcal/mole, which is intermediate between that of H2 (104 kcal/mole) and I2 (36 kcal/mole). Consider the gas-phase equilibrium reaction: A. Calculate the value of Bond breaking requires absorption of energy (endothermic, positive DH) and bond forming releases energy (exothermic, negative DH)
B. Present an argument why the value of DS for this reaction should be close to zero. The reaction is all in the gas phase. There are two moles of gas producing two moles of gas. As a result, the order of the system remains fairly constant and DS is approximately 0. C. At room temperature (T = 300 K) HI gas 1 atm pressure in a quartz vessel is found to be stable with respect to disproportionation to H2 and I2, i.e., the concentration ratio [I2]/[HI] = [H2]/[HI] is less than 1%. Is this observation a consequence of thermodynamics or of slow kinetics? Discuss. Useful information: R = 1.987 cal šK-1 mole-1.
However,
This reaction is not at standard conditions. Q ‚ 1 and DG ‚ DG°. Therefore, we can't use DG° as a criterion of spontaneity. We must use DG.
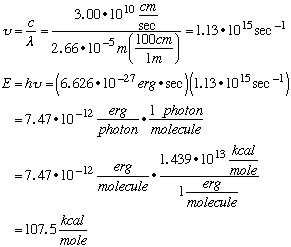
DG < 0, so this reqction is spontaneous in the forward direction. Therefore, the products are thermodynamically favored. D. When HI gas in the quartz vessel is irradiated with ultraviolet light at a wavelength of 266 nm it decomposes into atoms:
where v is the frequency of the light and h = 6.626 x 10-27 erg sec is Planck's constant. Calculate in kcal/mole how much energy is released to the surroundings (negative DH) per mole of HI decomposed to H + I at 266 nm. Some useful information: E = hn, c = 3.00 x 1010 cm/sec, 1 erg/molecule = 1.439 x 1013 kcal/mole.
107.5 kcal/mole – 71.0 kcal/mole (the energy required to break HI bonds) ––––––––––––––––––––––––––––– 36.5 kcal/mole=DH
At equilibrium, DG = 0 and Q = Keq, so
Therefore, the observed concentrations are not at equilibrium:
Q = 0.0001 << Keq = 0.35 So, the mixture is far away from equilibrium. Since the reaction is thermodynamically favored, it must be that a large kinetic barrier (high EA) is preventing the favored forward reaction. Slow kinetics is the cause for the concentrations in our systems. |