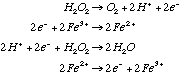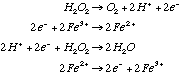Thermodynamics Solutions: #23
23.* (1994 3 4) When a trace of FeSO4 is added to an aqueous solution of H2O2, the solution foams; a gas evolves. When a trace of ZnSO4 is added to another aqueous solution of H2O2, no reaction occurs. A. Write equations showing the reactions that occur when FeSO4 is present:
B. Show that the reaction has a negative free energy change.
C. Explain the role of FeSO4 and why ZnSO4 has no effect. FeSO4 acts as a catalyst for this reaction but ZnSO4 does not. Only a trace of FeSO4 is required because, as a catalyst, it is regenerated. With a catalyst the first step is spontaneous:
With Zn:
|