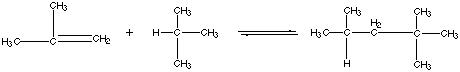Thermodynamics Solutions: #21
21.* (1994 F 11) Branched hydrocarbons burn more smoothly than "straight chain" hydrocarbons in automotive internal combustion engines. Thus to make "high-test" gasoline 2,2,4-trimethylpentane (iso-octane) is added to n-octane [CH3(CH2) 6CH3]. Today, large amounts of the anti-knock compound iso-octane are produced from the reaction of isobutylene and isobutane: At 25oC (298K) the gas-phase equilibrium constant is K = 4.3 x 10-6, and the heat of reaction (enthalpy of reaction) referenced to standard states is A. Offer and explanation for the sign of DHš. If we investigate what happens… (overall) C=C is converted to C-C and extra C-C bond is formed… The new C-C bond is the cause of the negative DH --> liberation of energy. B. Calculate DGo at 25oC for this reaction. DGo= -RT ln K = -(8.314 J K-1 mol-1)(298 K) ln (4.3 x 10-6) = 30,615 J / mol DGo= 30.62 kJ / mol C. Calculate D So at 25oC for this reaction. DGš = DHo - TDSo DSš = (DHo - DGo) / T = (-78.6 kJ / mol - 30.61 kJ / mol) / 298 K = -0.3665 kJ K-1 mol-1 DSš = -366.5 J K-1 mol-1 D. Offer an interpretation for the sign of DSo. The reaction involves taking two molecules to form one product molecule…the order of the system therefore increases… negative So = decrease in disorder = increase in order 2 gas molecules --> 1 gas molecule! E. Assume that DHo and DSo are independent of temperature. With this approximation estimate DGo and hence the equilibrium constant K at 700oC. DGo = DHo - TDSo = -78.6 kJ / mol - 973 K (-0.3665 kJ / mol•K) DGo= 278 kJ / mol at 973 K K = exp (-DGo / RT) = exp ( (-278,000 J / mol) / (8.314 J / mol•K • 973 K) ) K =1.2 x 10-15 F. To what extent does the reation go to completion at 700oC compared to 25oC? K (700oC) = 1.2 x 10-15 K (700oC) = 4.3 x 10-6 The reaction is far more unfavorable at high temperatures than at low temperatures! Therefore at high temperatures, the reaction mixture consists of reactants and only a trace of products. G. The first attempts at making iso-octane industrially from isobutane and isobutylene were not greatly successful, but it was found that a higher yield of iso-octane was obtained by heating the reaction mixture to 7000C rather than operating at room temperature. Offer an explanation for this finding. Although the thermodynamics are worse at high temperature, the rate at which the reaction occurs if far greater than at 25oC. H. A method of choice for making iso-octane from isobutylene and isobutane is to react the latter in the presence of an acidic catalyst such as concentrated sulfuric acid (H2SO4) or hydrofluoric acid (HF). This catalyzed process is typically carried out at 00C — 100C. Explain how the catalyst affects the value of the equilibrium constant for this reaction. The catalyst (as all catalysts) does NOT affect the value of the equilibrium constant; it only affects the kinetics of the process, not the thermodynamics. The catalyst lowers the activation energy barrier so that the reaction can proceed without excessive heat. |