Thermodynamics Solutions: #8
8.* (1996 F 15) The dimerization of nitrogen dioxide results in measurable amounts of reactant and product molecules at 250C. The reaction is: 
A. Write an expression for the equilibrium constant K. 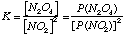
P(N2O4) = partial pressure of N2O4 (g) in atm. P(NO2) = partial pressure of NO2 (g) in atm. B. The value of K at 250C is 8.8. A mixture of the two gases is sampled and the partial pressures are found to be 9.8 x 10-2 atm for NO2 (g) and 2.1 x 10-1 atm for N2O4(g). Is this system at equilibrium? If not, what will be the spontaneous direction of change? At these conditions,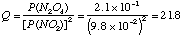
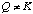 so the system is not at equilibrium. Since Q > K, the reaction will proceed toward equilibrium by going to the left, forming more NO2 (g) and less N2O4 (g). C. What is the sign of DH°? 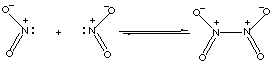
More bonds are formed than broken, so heat is given off. The reaction is exothermic, and DH°<0. D. What is the sign of DS°? We are forming 1 gas molecule from 2 gas molecules. Hence, there is more order in the products. 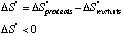
E. Predict how the equilibrium constant will change with temperature. 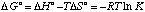
As T increases, unfavorable entropy (-TDS° > 0 because DS° < 0) takes over, and DG becomes more positive. Therfore, K decreases with higher temperature. 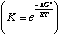
At low temperatures, -TDS is not as important as the favorable DH°> 0, so K increases with decreasing temperature. |