Thermodynamics Solutions: #5
5.* (1996 F 9) Consider the gases N2O, O2, and O3. Half-cell potentials are given for reduction of these substances in acid medium. 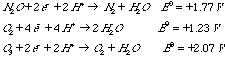
A. Arrange the substances in order of decreasing oxidizing power. High oxidizing power means the substance is easily reduced (has a high reduction potential) If the reduction potential is higher, it can oxidize more things. thus: O3 > N2O > O2 B. N2O ("laughing gas") is used as an anesthetic. It is rather inert and poses no danger of explosion in the operating theatre. For example, N2O does not react with H2. Is this what you expect based on the above potentials? Explain. Explosions only occur when an easy kinetic pathway exists (for example, a chain reaction.) N2O should be able to oxidize H2 or O2 in acid solution, since the reduction potentials for these species are less than that of N2O, but kinetics may prevent this from happening. Clearly the reactions don't occur in the gas phase under these conditions. So, the potentials may lead one to believe that the reactions would occur, but there's more to the story. |