Thermodynamics Solutions: #4
4.* (1997 3 3) An important class of enzymes, the cytochrome P450 family catalyzes the reaction between molecular oxygen and hydrocarbons producing alcohols. It may seem surprising that these enzymes use a 2e- reducing agent to activate oxygen such that the overall stoichiometry is as follows: 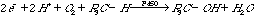
In fact, it is often found that oxidation reactions using oxygen also involve a 2e- reducing agent. You may find the following standard reduction poentials for oxygen (given in volts under standard conditions: 250C, [H+]=1M, [H2]=1 atm) to be useful. 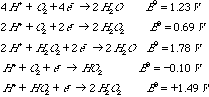
A. Explain how a reducing agent can make oxygen into a more powerful oxidant. Use thermodynamic arguments. A partial reduction of O2 can produce a form of oxygen which is a stronger oxidizing agent, like H2O2 (from a 2e- reduction of O2) or HO2 (from a 1e- reduction of O2). This is what is meant by the statement that molecular oxygen is activated by a 2e- reducing agent. B. The protonated form of superoxide ion, HO2, is unstable; it disproportionates for form hydrogen peroxide and oxygen. Show that this reaction is thermodynamically downhill under standard conditions. 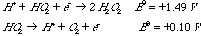
The sum of the standard potentials is +1.59V, so the reaction is spontaneous. Note that you must couple these two reactions (because we are told that it is a disproportionation) even though you could get the same net reaction in other ways. C. Three oxidation states of molecular oxygen (O2) are shown in the table of reduction potentials. Some of these are paramagnetic. Place an asterisk on those paramagnetic species. O2 has 2 unpaired electrons and is therefore paramagnetic. HO2- has one more electron since it is the one electron-reduced form of O2. This pairs with one of the 2 unpaired electrons of O2 and leaves the final species paramagnetic. H2O2 has two more electrons than O2, so that the pi* orbitals have no unpaired electrons. Thus it is diamagnetic. |