Thermodynamics Solutions: #3
3.* (1997 F 14) The following battery (galvanic cell) is constructed. 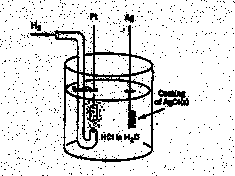
This battery may be regarded as the combination of two half cells: H+ (aq) + e- = 1/2 H2(g) E0 = 0.00V AgCl(s) + e- = Ag(s) + Cl- (aq) E0 = 0.22V Where the concentrations for H+(aq) and Cl-(aq) are 1M, the H2 (g) pressure is 1 atm, and the temperature is 250C. Actually, an extremely careful study has been made of this battery reaction by R. G. Bates and V. E. Bower, who measured the standard electromotive force E0 in volts as a function of temperature t in degrees centigrade. Their data may be represented as follows. E0/V = 0.23659-4.8564 x 10-4 (t/0C) — 3.4205 x 10-6 (t/0C) 2 + 5.869 x 10-9 (t/0C) 3 A. Use this data to find DG0 for the reaction 1/2 H2(g) + AgCl(s) --> H+ (aq) + Cl- (aq) + Ag(s) recalling that the Faraday constant is 96485 coulombs/mole, the universal gas constant R=8.314J/Kmol, and 1 coulomb-volt = 1Joule. Plugging 250C into the equation for E0, we obtain E0 = .2224V at 250C. Then plug this into DG0=-nFE0 where F is the Faraday constant we obtain —21.458 kJ/mol. B. Write down an expression for the eqilibrium constant K at 25oC for this reaction and find the value of K. Solving for K in DG0=-RTlnK and plugging in the values for DG0 and R above, we obtain K = 5.77 x 103. C. Use the data provided to find DS0 for this battery reaction. 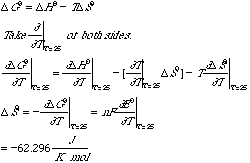
D. Under standard state conditions is this battery reaction driven by entropy change, driven by enthalpy change, or driven by both entropy and enthalpy changes? Explain. Since entropy change is negative, -TDS0 is positive, so for the reaction to proceed spontaneously as written it must be enthalpically driven. |