Thermodynamics Solutions: #2
2.* (1997 F 11) Adenine triphosphate (ATP) is the principal immediate donor of free energy in biological reactions. In a cell, ATP is being formed and consumed all the time; the typical delay between formation and consumption is approximately one minute. The turnover rate of ATP is remarkably high. A resting human consumes about 40kg of ATP in 24 hours, which is an appreciable fraction of the total body weight! A large amount of free energy is liberated when ATP is hydrolyzed to adenine diphosphate (ADP) and orthophosphate (Pi). ATP + H2O ---> ADP + H+ +Pi DG0 = -16.8 kcal/mol To understand the role of ATP in free energy coupling, consider the reaction X ---> Y DG0 = +7 kcal/mol A. Calculate the equilibrium constant K = [Y]/[X] for this reaction. Use R = 2cal/Kmol. [Y]/[X] = e(-7000/(2*298)) = 7.93 x 10-6 The extremely small value of this equilibrium constant tells us that at equilibrium, there is very little Y and a lot of X. Therefore X is the more stable form. B. Thus X cannot be spontaneously converted to Y when the molar ration of [Y] to [X] is equal to or greater than K. But X can be converted to Y when the [Y]/[X] ration is coupled to the hydrolysis to ATP. The new overall reaction is X + ATP + H2O --> Y + ADP + H+ +Pi DG = -9.8 kcal/mol Where the value of DG0 for this reaction is the sum of the DG0 values for the two separate reactions. Under the conditions of a coupled reaction K’ = ([Y]/[X])([ADP][Pi][H+]/[ATP]). Find the value of K’. Room temperature is 298K; body temperature is 310K. Either temperature was appropriate to be used in the subsequent calculations. Room temp: K’ = e-(-9800/(2*298)) = 1.38x 107 = K’ Body Temp: K’ = e-(-9800/(2*310)) = 7.32 x 106 = K’ C. The ATP-generating system of cells maintains the [ATP]/([ADP][Pi]H+] ratio at a high level, typically on the order of 500. Use this information to calculate [Y]/[X] for the coupled reaction. K’ = ([Y]/[X])([ADP][Pi][H+]/[ADP]) ---> [Y]/[X] = K’*[ATP]/([ADP][Pi][H+]) = 500K’ = 6.9 x 109 @ room temp = 3.66 x 109 @ body temp D. By comparing your results for parts A and C, determine by how much the hydrolysis of an ATP molecule changes the equilibrium ratio of [Y]/[X] when it is a coupled reaction compared to an uncoupled reaction. At room temp: 6.9 x 109/7.93 x 10-6. At body temp: 3.66 x 109/7.93 x 10-6. The ratio increases by a lot!! E. Here is a system analogous to metabolic thermodynamics. Suppose compound A is broken down into E by the following three-step reaction sequence:
What is the standard free energy change for the reaction A ---> E? D G0 = -3 - 55 - 32 = -90 kcal/molF. Coupled to the second step is the reaction F -> G for which DG0 = +60 kcal/mol. Explain how it is possible that a reaction with DG0 of 60 kcal/mol can be made to go by one of only —55 kcal/mol.Equimolar quantities of C, D, F, and G are not necessarily involved in the reaction. G. Assuming equimolar concentrations of F and G, what must be the relative concentration of C and D for perfect free energy coupling between C ---> D and F ---> G reactions, that is, so that the overall DG0 value is zero?There were three responses that received full credit for this part.
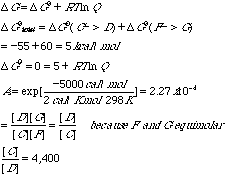
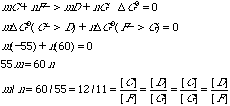
H. Demonstrate rigorously that the total free energy change in one turn of the cycle is independent of the concentrations of B, C, and D. 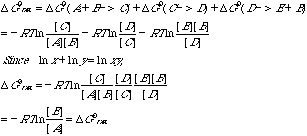
|