Thermodynamics Solutions: #19
19.* (1995 3 5) A. A weight is hung from the ceiling with a rubber band. As the temperature of the room changes describe what happens to the length of the rubber band. Explain. long rubber band --> short rubber band DS>0 -- more conformations are possible for a relaxed polymer As a rubber band is stretched, it gives off heat. Thus, as the room temperature increases, the rubber band will contract due to Le Chatelier's principle. This is straight from Professor Zare's rubber band handout. "The force exerted by a stretched rubber band is almost completely entropic in origin. The force is also proportional to the temperature. If rubber band is heated at constant force, it will contract!" B. According to thermodynamics a reaction system always proceeds to equilibrium at which the concentration of the reagents and products have a fixed relation described by the equilibrium constant. Suppose the initial concentration of reagent A denoted by [A]t=0 is much larger than the concentration of A at equilibrium denoted by [A]t=infinity. Does the time dependance of the concentration of A denoted by [A]t behave such that [A]t>[A]t+Dt where Dt is a positive increment of time, i.e. is the approach of the concentration of a reagent A to equilibrium always monotonically decreasing if 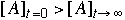 ? ?
Thermodynamics tells nothing about kinetics. Thus, while [A] must go to its equilibrium value eventually, it may not go there directly. For example, the following is possible: 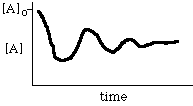
|