Thermodynamics Solutions: #18
18.* (1995 3 3) Chemists often rely on the rough rule that "like dissolves like" in estimating the solubility of a substance in a solvent. According to this concept, polar solvents dissolve polar substances and nonpolar solvents dissolve nonpolar substances, but solution is not favored for a polar solvent and a nonpolar substance or a nonpolar solvent and a polar substance. You are asked here to present a thermodynamic argument why like dissolves like but like does not dissolve well unlike. To be specific, consider that the process of dissolution of a crystalline substance in a solvent takes place when the substance is placed inside a beaker containing the solvent and that solution takes place at essentially constant temperature T and pressure P. To help you make this argument, answer the following questions. A. Define for this process the system (sys) and the surroundings (surr). sys = solvent and substance surr = rest of universe beaker can be considered part of either sys or surr B. What are the conditions in terms of źS and źG for solution of the substance in the solvent to proceed spontaneously?
(DG gives chemists a convenient way to check thermodynamics of a reaction or process--there is no need to look at the rest of "the universe") C. What is the sign of DSsys when (a) like dissolves in like and (b) like dissolves in unlike? 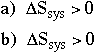
In both cases, the dissolution increases the disorder of the system. D. What is the sign and estimate approximately the magnitude of źH when (a) like dissolves in like and (b) like dissolves in unlike. 
(because you're breaking like-like interactions between the substance's molecules, and replacing them with similar like-like interactions between solid and substance.) 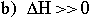
(because you're breaking favorable like-like interactions between the substances molecules and replacing them with unfavorable like-unlike interactions. It takes energy to do this.) E. Use the above answers to provide a thermodynamics basis for this chemists’ rule of thumb. For like-like: 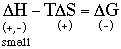
For like-unlike: 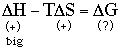
Thus, since the entropies are similar, the źH terms are what differ and cause 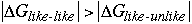
F. Even if like does not dissolve unlike so well as like dissolves like, explain why increasing the temperature of the solvent generally increases the solubility of like into unlike. For like-unlike: DH > 0, DS > 0, thus as T increases, -TDS term dominates and the reaction is entropically driven so that DG --> negative as T increases. |