Thermodynamics Solutions: #13
13.* (1995 F 3) For the following pairs, which has the higher entropy or more positive entropy change? A.
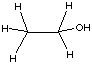
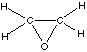
Ethanol (a) has more conformations than (b), because the ring in (b) is more ordered. B. 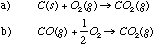
Reaction (a) has a higher entropy change because a mole of solid (highly ordered) is reacting to form a highly disordered mole of gas. In reaction (b) źS is actually less than 0 because 1.5 moles of gas are becoming 1.0 mole of gas. C. 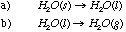
(b) has a higher entropy change because the liquid-gas transition involves a higher increase in disorder than a solid-liquid transition: Disorder: solid < liquid << gas |