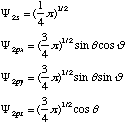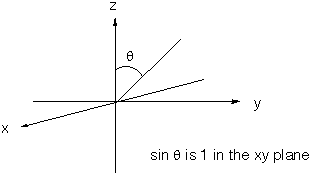Structure and Bonding Solutions: #99.* (1993 1 2) The angular parts of the 2s and 2p atomic orbitals on the carbon atom may be written as
The radial parts of these orbitals may be treated as the same. Consider a sp2 hybrid orbital composed of a linear combination of one 2s atomic orbital and two different 2p atomic orbitals. Present an argument why the three different sp2 hybrid orbitals have their maximum bonding power in a plane. As we know, the three different sp2 hybrids point to the corners of an equilateral triangle. The maximum bonding power will occur where the three orbitals (2s, 2px, and 2py) have their maximum overlap. Since Y(2px) and Y(2py) both have maximum electron density in the xy plane (where sinq is 1), the maximum overlap (and therefore maximum bonding power) will occur in the x-y plane.
Note: Three orbitals define a plane, so you can choose (2s, 2px, and 2pz) , (2s, 2py, and 2pz), or (2s, 2px, and 2py). A similar argument can be applied for all three sets of orbitals. An alternative solution relies on the fact that one p-orbital is unhybridized and must be orthogonal to the sp2 hybridized orbitals. Therefore, the sp2 orbitals must lie in a plane perpendicular to the unhybridized p-orbital. |