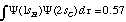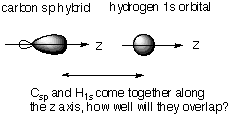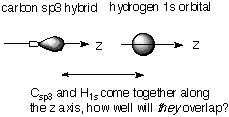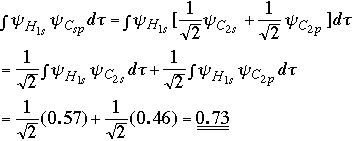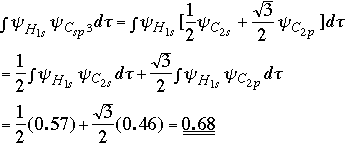Structure and Bonding Solutions: #88.* (1994 1 2) The C-H bond in ethyne (C2H2) is known to be stronger and stiffer than the C-H bond in CH4, i.e., the C-H vibrational stretching motion in ethyne is at a higher frequency than that in CH4. This problem explores whether these facts can be understood on the basis of simple hybridized orbitals of carbon. The question we wish to answer is whether the constructive overlap of the H 1s atomic orbital Y(1sH) with the C sp hydrid orbital of ethyne puts more electron charge between the C and H centers than the overlap of the H 1s atomic orbital with the C sp3 hybrid orbital of CH4. Note that the overlap squared is proportional to the probability of finding the charge between the two nuclei. The overlap integral between hydrogen 1s and a carbon 2s orbital separated by the C-H bond length is 0.57, i.e.,
where dt = dxdydz is the inifnitesimal volume element. The overlap integral between the hydrogen 1s orbital and the carbon 2pz orbital is 0.46, i.e.,
The sp hybrid orbital that points along the z axis is a linear combination of the C 2s and 2pz orbitals
whereas the sp3 orbital directed along the z axis is
Calculate the overlap integral between the hydrogen 1s and the carbon sp and sp3 hybrid orbitals directed along the z axis, which is taken to coincide with the C-H bond, assuming that the C-H bond distance of both ethyne and methane are the same. Does your result show the expected behavior? If not, can you suggest a reason for this failure? The question in qualitative terms is asking: which is better, the overlap between an sp hybrid along the z axis and H 1s or the overlap between an sp3 hybrid along the z axis and H 1s.
Notice from the picture that the sp3 hybrid has less electron density along the z axis. This is because it has some px and py mixed in as well as pz. Because of this, we expect the sp hybrid, which focuses its full electron density (i.e. maximum bonding power) along z, to have the best overlap with H1s. Now that we understand the problem, all we have left to do is the math . . . Overlap for bond involving Csp orbital:
Overlap for bond involving Csp3 orbital:
The Csp-H bond has a stronger overlap and would therefore be expected to have the stronger bond. |