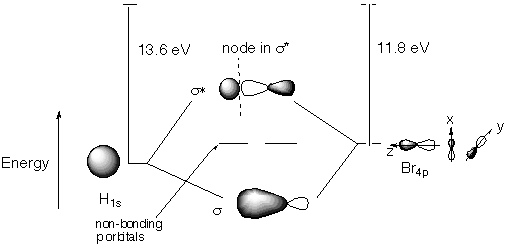
Structure and Bonding Solutions: #77.* (1995 1 5) The ground state electron configuration of the H atom is that of a single electron in the 1s atomic orbital. The ground state configuration of the Br atom is 1s22s22p63s23p64s23d104p5. A. Draw an energy level diagram showing what s and p molecular orbitals result from the linear combination of atomic orbitals obtained by combining H (1s) and Br(4p) and their relative energy ordering. Order the s and p molecular orbitals in energy with the most bonding on the bottom and the least bonding on the top. Show their energies relative to the energies of the H(1s) and Br(4p) atomic energy levels. In this regard, please note that the ionization energy (the energy to remove an electron from the topmost level to form the ion) is 13.595 eV for H(1s) and 11.84 eV for Br(4p).
Note that we can disregard the Br core (its 1s-4s orbitals) because we are told in the statement of the problem that only the H 1s and Br 4p orbitals have energies that are similar enough to allow for interaction. The p orbitals that do not have C-infinity symmetry around the internuclear axis cannot bond with H1s: their overlap with the H 1s orbital cancels. The only p orbital of requisite symmetry to bond with H 1s is Br 4pz (assuming we take z to be the internuclear axis). C. What is the multiplicity of HBr? All electrons are paired, so multiplicity = 2S + 1 = 2(0) + 1 = 1. HBr is a singlet. D. Is HBr paramagmetic or diamagnetic? HBr is diamagnetic. All electrons are paired, so the sample of HBr will be repelled by a magnetic field. E. The ionization potential of HBr is 11.67 eV, which is nearly the same as that of Br (11.84 eV). Offer an explanation for this behavior. The ionization potential is a measure of how much energy it takes to remove an electron from the highest occupied molecular orbital or highest occupied atomic orbital. From the MO diagram, we see that all four electrons in the p non-bonding orbitals come from the Br’s 4p orbitals. The energy of the nonbonding HBr molecular orbitals is essentially the same as the 4p atomic orbitals in Br. Thus it takes about the same amount of energy to knock an electron out of HBr as it does to knock one out of Br. |