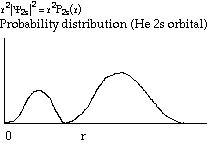
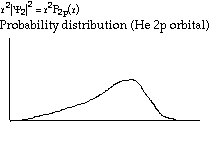
Structure and Bonding Solutions: #66.* (1996 1 1) The electronic ground-state configuration of the hydrogen atom is one electron in the 1s orbital, denoted by H(1s). The electronic ground-state configuration of the helium atom is two electrons in the 1s orbital, denoted by He(1s2). The ionization potential of H(1s) is 13.6eV and that of He(1s2) is 24.6 eV. A. Explain why the ionization potential of He(1s2) is greater than that of H(1s). He has more protons in its nucleus. These protons suck the electrons in tight via coulombic attraction and make the electrons harder to pull off. B. The lowest-lying excited electron configurations of the H atom are H(2s) and H(2p), which have identical energies. For the He atom, however, the lowest-lying excited electron configurations are He(1s2s) and He(1s2p). They are not at the same energy. Instead, the energy of He(1s2s) lies lower than that of He(1s2p), that is, the electron in the 2s orbital in He is mor tightly bound than the one in the 2p orbital. Offer an explanation for this. In He, the existing electron in the 1s orbital shields the n=2 electrons from the pull of the nucleus. However, it shields those n=2 electrons differentially; the electron density of the 2s orbital is distributed closer to the nucleus than that of the 2p orbital and therefore is less effectively shielded. Hence the electron in the 2s orbital feels a greater pull from the nucleus than does an electron in the 2p.
C. The He(1s2p) electron configuration actually has two energy levels: He(1s2p) 1P and He(1s2p) 3P. Explain what is the difference between these two atomic energy states. The singlet P state is one where the two electrons have opposite spin, and the triplet P is one where the two electrons have the same spin. D. Predict the energy ordering the the He(1s2p) 1P and 3P energy states and explain the reasoning behind your prediction. The singlet P state is higher in energy (less favorable) because higher multiplicity is always lower energy. |