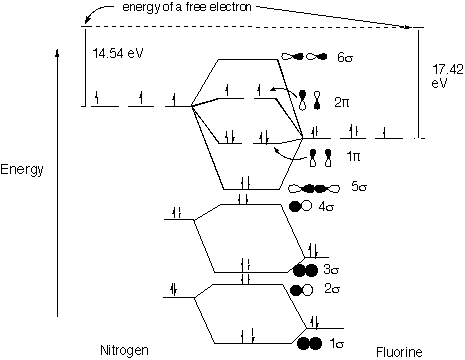
Structure and Bonding Solutions: #44.* (1997 1 7) Consider the diatomic molecule NF. Draw its molecular orbital energy diagram.
A. Using the LCAO-MO scheme, indicate the ground-state MO description for NF, i.e. complete the following: 1(s)2 . . . The LCAO-MO description is: 1s22s23s24s25s21p42p2. B. What is the multiplicity of the ground state of NF? In the ground state, NF has 2 unpaired electrons, which gives it a spin S of 1 and a multiplicity (multiplicity = 2S + 1) of 3. This makes it a triplet. C. Do you expect NF to be diamagnetic or paramagnetic? Why? NF is paramagnetic because of its two unpaired electrons. D. The ionization potential of N is 14.54 eV and that for F is 17.42 eV. Do you expect the ionization potential of NF to be greater than that of F, closer to N than F, closer to F than N, or less than N? Explain. Its ionization potential is less than N. The 2p MO is more N-like than F-like in character (because it is closer in energy to N) and its energy is raised (antibonding) so that it is easier to ionize. |