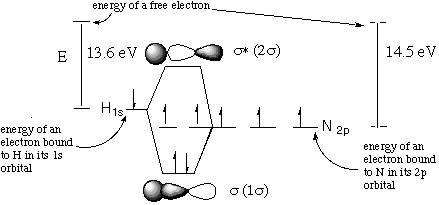
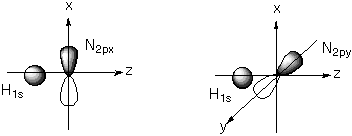Structure and Bonding Solutions: #33.* (1997 F 10) The NH radical is a highly reactive gas-phase species. Use the LCAO-MO theory to describe its electronic structure. Here the H(1s) atomic orbital is combined with the N(2p) atomic orbital. The ionization potential of H 1s is 13.6 eV and that of N 1s22s22p3 is 14.5 eV. Answer the following questions: A. Draw an energy level diagram of the MO’s in NH arising from combining the 1s atomic orbital of H with the three 2p atomic orbitals of N. Indicate how the four valence electrons of NH occupy these orbitals.
Though this part of the problem doesn't require pictures of the orbitals or labelling of the ionization energies of H and N, they are added to the diagram for clarity. Remember the meaning of ionization energy: the fact that it takes 13.6 eV to ionize H means that the energy of an electron in H is 13.6 eV lower than the energy of a free electron. This comes about because the electron is stabilized by its attraction to the H nucleus. Since the N nucleus has more protons, the attraction is greater so it makes sense that an electron bound to N is stabilized more than an H electron. Therefore the ionization energy of N is higher. B. What is the multiplicity of NH in its ground electronic state? Do you expect NH to be paramagnetic or diamagnetic? S = 1. multiplicity = 2S + 1 = 3. Triplet. With 2 unpaired electrons, we expect NH to be paramagnetic. C. Draw pictures of the s and s* orbitals of NH showing the charge distribution relative to the N and H atomic centers.
Y *Y=|Y2|
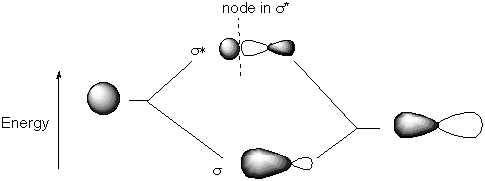
The s (1 s) and s * (2 s) molecular orbitals were drawn in the MO diagram above, but here they are shown more explicitly. Note the nodal plane in the high energy, antibonding combitation of the orbitals. D. Draw pictures of the valence orbitals of NH showing the charge distribution relative to the N and H atomic centers. The valence orbitals are the non-interacting N 2p orbitals. The position of the H is shown here for location only. By convention, the internuclear axis is taken as z.
E. The ionization potential of NH has been determined to be 13.63eV. Is this value expected by you based on LCAO-MO theory? Explain. No! We expect 14.5 eV, since the highest occupied valence orbitals are the non-interacting orbitals on N. (See the diagram for part A.) Evidently, the s orbital on the N atom helps to screen p electrons and therefore makes it easier to remove one of them. |