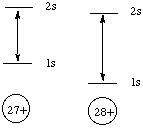
Structure and Bonding Solutions: #22. Before 1913, scientists were unable to measure the atomic numbers of elements directly. Therefore, the elements of the periodic table were ordered by their atomic masses. A. In some cases, the ordering of elements by atomic mass does not match their order by atomic number. Why? Recall that atomic masses are the weighted average of the masses of the various isotopes of the element. Neutrons in the nucleii of atoms affect the masses but not the atomic numbers of the elements. For example, cobalt has an atomic number of 27 and an atomic mass of 58.9, while nickel has an atomic number of 28 and an atomic mass of 58.7. B. X-rays are emmitted when an electron drops from the 2s orbital of an atom to that atom's 1s orbital. Such radiation is called "Ka x-ray radiation." Which would have a larger energy, the Ka x-rays emmitted by Co or the Ka x-rays emmitted by Ni? Briefly explain your answer. Ni has atomic number 28, meaning that there are 28 protons in its nucleus. Co, with atomic number 27, has slightly fewer protons in its nucleus. The 1s electrons, being closest to the nucleus, feel this nuclear charge difference the strongest. They will be sucked in tighter to Ni than to Co, as shown in the energy diagram below. Note that the 2s electrons will also feel the nuclear charge difference, but not nearly to the same degree that the 1s electrons do.
The energy difference between the 1s and 2s levels is higher for Ni, so the Ka x-rays emmitted by Ni will have a greater energy than the Ka x-rays emmitted by Co. C. In 1913, a young British scientist named Henry Mosely used Ka x-ray emmission to put the elements of the periodic table into their proper order by atomic number. How did he do it? Why didn't atomic mass make a difference? The energies of the Ka x-rays emmitted by the elements increase with increasing atomic number, so Mosely simply put the elements in order by their Ka x-ray energies. The isotopic differences in atomic mass don't matter because these arise from differences in numbers of neutrons, which are not charged. |