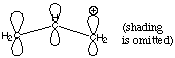Structure and Bonding Solutions: #11. Imagine we have three atoms of sp2-hybridized carbon side by side. The unhybridized p orbitals of these atoms are parallel to each other. One of the carbons does not have four bonds, but instead has a positive charge located in its unhybridized p orbital. This configuration is called an "allyl cation."
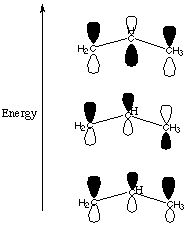
A. Why is the positive charge located in the unhybridized 2p orbital instead of one of the sp2 orbitals? The positive charge is repelled by the positively charged nucleus. Since s orbitals are closer to the nucleus than p orbitals, the positive charge localizes in an orbital with the least s character available: in this case, a pure p orbital. B. How many electrons are located in the three p orbitals (the p system) depicted above? There are two electrons in the p system. Remember that carbon has 4 valence electrons, so one electron goes into each of the sp2 hybrids and then the last one is left over to fit into the p. That gives us a total of three, and after we subtract one to account for the positive charge, we are left with two. C. Ignoring the skeletal framework of this molecular ion and the position of the positive charge, add and subtract the wavefunctions of the three p orbitals to construct a molecular orbital diagram describing the situation. Be sure to give your molecular orbitals the correct energetic order.
Notice that since we started with three atomic p orbitals, we had to come up with three molecular p orbitals. The number of contributing atomic orbitals always equals the number of orbitals you end up with. The best p-type overlap is exhibited by the lowest energy (most bonding) orbital. In the middle orbital there is one nodal plane and in the highest energy (most antibonding) orbital there are two nodal planes. D. Each of your molecular orbitals can accomodate two electrons. Which orbitals are filled? What does this tell you about the electron density in this allyl cation system? We start with two electrons in the p system as explained in part B. Therefore only the lowest energy molecular orbital in part C is filled. This tells us that the electron density is spread out over all three of the unhybridized p orbitals. E. Where is the positive charge in the allyl cation? Just like the electron density, the positive charge in this system is spread out over all three carbons in this system. We will study this phenomenon in the section on organic chemistry and call it "resonance." The fact that the electron density and the positive charge are spread out over the whole p system has vast repurcussions for organic reaction chemistry. |