Stereochemistry Part 2 Solutions: #17
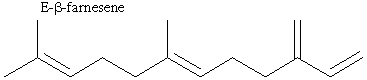
17.* (1990 F 9) E-b-farnesene, the aphid alarm pheromone, is produced in the leaves of the Bolivian potato which is not attacked by aphids.
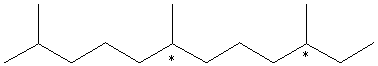
A. Is it chiral? This compound is achiral. It has no chiral centers and it has a mirror plane in the plane of the page. B. Exhaustive hydrogenation saturates all of th olefinic bonds but no skeletal rearrangement occurs. Write structural formulas for the products of hydrogenation and comment on optical activity.
This compound has 2 stereocenters which are marked. Each of these can be R or S, so there are 4 stereoisomers: RS, RR, SR, and SS. Hydrogenation is not stereospecific, so all of these stereoisomers will be present in equal amounts, creating a racemic mixture with no optical activity. C. Is there any conjugation in this molecule? Conjugation in E-b-farnesene is circled above. D. Where does the "E" come from? E is an abbreviation of the German "Entgegen." The middle double bond can have two conformations. The higher-priority (longer) carbon chains can be on the same or opposite sides of the bond. In this case they are opposite. Entgegen means "opposite," and Z (Zusammen) means "together." |