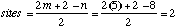Stereochemistry Part 2 Solutions: #11
11.* (1995 1 3) Consider a hydrocarbon, X (molecular formula C5H8) which is optically active. This substance manifests all three hybridization states of carbon (sp, sp2, and sp3). Reaction of X with H2 over Pd metal catalyst forms n-pentane. Such reactions do not break carbon-carbon bonds. A. How many sites of unsaturation are in X? B. Draw three dimensional structural formulas for X and for any stereoisomers of X. Can you distinguish between the stereoisomers using the information given here?
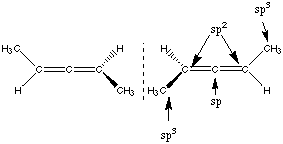
These are enantiomers, but they cannot be distinguished based on the given information. C. Are there any chiral carbon centers in X? No. This is a chiral molecule without chiral centers. D. Indicate the hybridization states of each carbon in X by drawing an arrow from the symbols, sp, sp2, and sp3 to these carbon atoms. Hybridization states indicated above. |