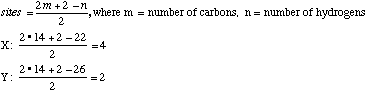Stereochemistry Part 1 Solutions: #9
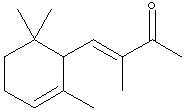
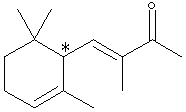
A sample X (Xn) derived from natural flower oils is optically active; however, a sample of X (Xs) produced from a total synthesis does not rotate plane-polarized light even though this synthetic sample, Xs, exhibits both an infrared spectrum and 1H and 13C-NMR spectra identical to those of the sample Xn derived from natural sources. The natural and synthetic samples Xn and Xs have different fragrances. Catalytic hydrogenation of one mole of either Xn or Xs over a Pd catalyst took up to 2 moles of H2 (as indicated below). The product Ys from Xs was shown to be a mixture of 4 components. When the 4 components of product Ys were separated with a gas chromatograph, each component showed the same molecular formula (C14H26O) as determined by mass spectrometry. Xn, Xs, and the 4 components of Ys show a strong infrared peak in the region associated with a ketone (1690-1700 cm-1). On the basis of this information answer the following: A. How many sites of unsaturation are in X and Y, respectively? use
Or, you could count sites, including C=C bonds, C=O bonds, and rings. B. Write an asterisk beside each chiral center in the structural formula shown for X.
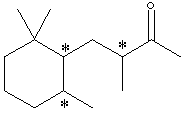
C. Identify the nature of the apparent stereoisomers: Xn and Xs. Xn is an optically pure chiral compound (one enantiomer.) Xs is a racemic mixture of Xn and its enantiomer. Synthesis with achiral reagents will always give optically inactive products. If the products are chiral, they will be racemates. Although the problem does not state whether the reagents were chiral, the fact that Xs and Xn have the same NMR and IR spectra means that Xs can’t just be a diastereomer of Xn. The fact that it is optically inactive means it’s not just an enantiomer of Xn. This leaves only a racemic mixture of Xn and its enantiomer. D. Write the structural formula for Y in chickenwire notation. see below E. Place asterisks beside each chiral center on your structure for Y.
F. What is the nature of the four stereoisomeric components represented by Y? A simple term will do. Gas chromatography does not separate enantiomers. Thus each component is a pair of enantiomers which is a diastereomer of each of the other pairs.G. Using the terms R and S, explain why Yn would exist as 4 stereoisomers. Yn should be the product of catalytic hydrogenation of Xn. Yn has 3 chiral carbons, but one of them will have the same absolute configuration as it did in Xn. The other 2 carbons will be evenly distributed in R and S, allowing for 4 diastereomers, each of which is one enantiomer of a pair whose other enantiomer is not produced: XRR, XRS, XSR, XSS. Also acceptable for full credit: Since stereoisomer has been used interchangeably with separated component in this problem, one may have interpreted the problem as asking why 4 components were separated from Ys. This is because Ys has 3 chiral carbons and thus 23=8 stereoisomers, but only 4 enantiomeric pairs: RS RS RS RS RS RS SR SR RS SR RS SR |