Stereochemistry Part 1 Solutions: #7
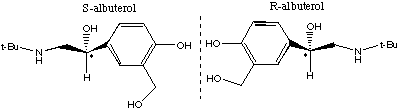
7.* (1995 F 4) According to the Wall Street Journal (Nov 7, 1995) "many drugs have the personalities of Dr. Jekyll and Mr. Hyde: they relieve pain but also upset the stomach." By separating the "good molecules" from the "evil" twins, researchers can reduce the negative side effects of some drugs. A case in point is S-albuterol (the bad twin) and R-albuterol (the good twin). The former increases the severity of asthma attacks whereas the latter relaxes bronchial muscles. Structures of S- and R- albuterol are shown below in chicken-wire notation.
A. Explain the different physiological effects of such twins. The twins are enantiomers. (The CH2OH groups are shown in different conformations.) Various cell receptors are chiral and have very different affinities for the two different enantiomers. In some cases, one enantiomer may be beneficial while the other is harmful. B. Discuss some physical properties of the twins. Which would be the same and which would be different? Enantiomers have very similar properties:
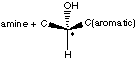
C. How did these twins get their names "S" and "R"? They got their names by the absolute configuration of the sterocenter. The priority assignment here is a bit tricky. Look at what is attached to the chiral center on S-albuterol:
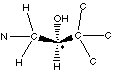
Which has the higher priority, the aromatic ring, or the amine? The amine. Remember, when prioritizing by atomic number, if two side chains are similar, go only until the first difference and no further. For this example, treat the C=C bond in the aromatic ring as if the carbon was bonded to two carbons. Therefore, we have the following:
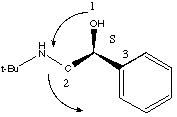
Putting the H in back, we get:
|