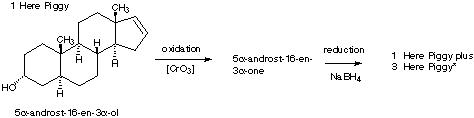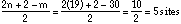Stereochemistry Part 1 Solutions: #6
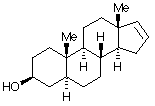
6.* (1996 1 3) Below is shown the structure of a naturally-occurring male sex pheromone found in pigs: 5a-androst-16-en-3 a-ol. This compound, called "Here Piggy," is said to have a musk odor, and roughly one-half of the humn population can detect this odor. Of those, about half find the odor attractive; the other half find it repulsive. Male pigs (boars) made and excrete Here Piggy from their saliva; this odor sexually arouses female pigs (sows). The same compound (Here Piggy) has been isolated from the delectable fungus, truffles. In France, pigs are used to detect truffles under the ground.
A. Write down the molecular formula for Here Piggy. C19H30O B. How many sites of unsaturation are there in Here Piggy? Identify them in a short statement. 5 sites of unsaturation: 4 rings and one ¼ bond or: C. Place an asterisk beside each chiral carbon on the structure shown for pheromone Here Piggy. How many stereoisomers could exist for this general structure? Show your calculations. 7 chiral centers, 27 = 128 possible stereoisomers because all the stereocenters are different.
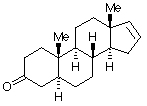
5- a -androst-16-en-3 a -olD. The pheromone Here Piggy can be oxidized into another compound, 5 a-androst-16-en-3-one, which has two fewer hydrogen atoms. Write a structural formula for this new substance by making use of your knowledge of nomenclature. When 5 >b>a -androst-16-en-3-one was treated with a reducing agent selective for carbonyl derivatives, two isomeric products were formed; one is identical to the original Here Piggy.oxidized form is:
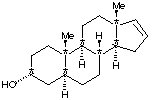
5 a -androst-16-en-3-one (-one ending indicates a ketone)E. Draw the a structural formula for the other isomer of Here Piggy showing the stereochemistry. A reducing agent selective for carbonyl derivatives will only attack at the C=O bond. No other stereocenters are affected by the oxidation or reduction. Because the reduction is not stereospecific, two isomeric products are formed. One is identical to structure #1, and the other is shown below:
F. What term describes the relationship between the original Here Piggy and its isomer? The two are diastereomers because the configuration of only one stereocenter was changed. G. Constrast the odor and optical activity you might expect to be manifest by these two isomers. optical activity: both are chiral, and both rotate plane-polarized light, but because they are not enantiomers, there is not necessarily a relationship between the magnitude or direction of their light rotation. odors: They will smell different. Diastereomers have different physical properties, as well as different biological properties (such as smell.) |