Stereochemistry Part 1 Solutions: #5
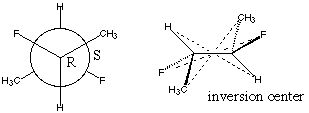
5.* (1996 F 6) The complete Newman formula for 2S, 3R difluorobutane is shown below, along with another diagram.
What is the relationship between this compound and 2R, 3S difluorobutane? They are the same compound. Since RS difluorobutane has an internal center of inversion (or mirror plane, depending on conformation), reflecting it will not change the stereochemistry, just the numbering of the carbons. C. Comment on the optical activity of these compounds. These "two" compounds (which are actually the same compound) are optically inactive. Since they have an internal center of inversion (or mirror plane, depending on the conformation) they are not chiral. Thus, they don't rotate plane polarized light. |