Stereochemistry Part 1 Solutions: #3
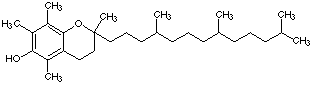
3.* (1996 F 4) Vitamin E is a fat soluble vitamin essential for muscle development; its structure is shown below. Two chemistry 32 students take Vitamin E; Fred gets his vitamin from a discount drug store; Sara believes in "natural foods" and so she buys her Vitamin E from a health food store. Fred’s Vitamin E is made in a factory; Sara’s Vitamin E is derived from soybeans. Fred and Sara compared their Vitamin E samples by taking a melting point and measuring the 1H NMR spectrum of each. The melting points are different!!
Vitamin E A. What is the difference between Fred and Sara's samples? Natural compounds, such as Sara's sample, occur as one enantiomer. Vitamin E has 3 chiral centers, so there are 23=8 possible stereoisomers, many of which are probably contained in the synthetic analog that Fred bought. B. What additional experiment would clarify the difference between Fred and Sara's Vitamin E? Because Fred's sample is a mixture of diastereomers and Sara's sample is one pure enantiomer, the samples will have different physical and light-rotating properties. Chromatography or solubility experiments could clarify the differences in physical properties. NMR might elucidate how the structures differ. In addition, the optical activity of the samples could be checked. (Fred's sample is probably racemic.) C. Would the NMR spectra of the two samples be the same? They would be different. Fred's NMR would be complex because he has a mixture of diastereomers, which would be present in unpredictable ratios; Sara's would be simpler. D. What do you advise Fred and Sara about taking the synthetic versus natural Vitamin E? Biological receptors are often stereospecific, so only the proper enantiomer would be effective. Fred's Vitamin E probably has only 1/8 of the proper compound and probably contains other diastereomers that may be harmful. Sara's Vitamin E is a pure sample of the proper, natural compound and would be well received in the body. |