Non-NMR Spectroscopies Solutions: #8
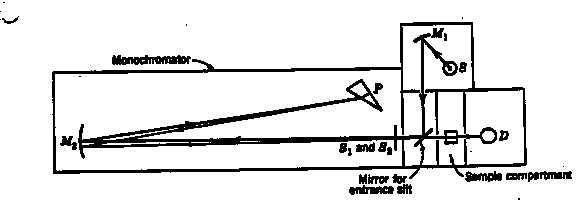
8.* (1994 2 5) Shown in the figure below is a typical absorption spectrophotometer. |
|
The source is a battery-operated tungsten lamp that provides a "white" light for operation in the visible. A mirror M1 sends a beam of light from this source through the entrance slit S1 into the monochromator. This beam is reflected to a quartz prism by the collimating mirror M2. The prism P is of the so-called Littrow type so that the light undergoes refraction on entering the front surface, is relected from the solvered back surface, and undergoes a second refraction on leaving the prism. When the prism is rotated, a narrow band of light, which is nearly monochromatic at any desired wavelength in the visible, can be passed throught the exit slit S2. A sample cell, with plane parallel windows, is mounted in the sample compartment between the exit slit and the photodetector D. All light that is transmitted by the sample falls on the detector whose photocurrent is amplified and recorded. The magnitude of the photocurrent is proportional to the intensity of the transmitted light. a. Suppose you were given an aqueous solution of Ru(bipy)32+ of unknown concentration [x] in units of moles/liter. Suppose also that you had available 10 g of pure Ru(bipy) 32+. Carefully explain what operations you would undertake to determine the value of [x]. Some possibly useful information follows: For Beer's law, I = Ioe(-ecl); Ru(bipy) 32+ has the chemical formula Ru(C10H8N2) 3, and the atomic weights are Ru = 101.07 g/mole, C = 12.011 g/mole, H = 1.008 g/mole, and N = 14.007 g/mole. To find the unknown concentration [x] in moles/L from the given information, first make a solution of known concentration. Then calculate e from the Beer’s Law equation for some wavelength l. Then run the same experiment in the same sample cell at the same wavelength on the unknown solution--now that you know e from the last run, you can calculate [x]. You will solve for the values in the following order: I = Ioe(-ecl) e = -(1/cl) ln [I/Io] (solution of known concentration c)[x] = -(1/ el) ln [I/Io] (unknown solution)
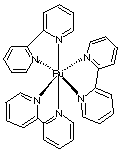
B. Prepare a set of instructions for a beginning student explaining how you would use the instrument shown in the figure to obtain an absorption spectrum of an aqueous solution of Ru(bipy)32+. 1. Perform a wavelength calibration of the spectrometer by using a standard solution of known concentration that absorbs known amounts at known wavelengths. This is to ensure that the spectrometer is calibrated. 2. Fill a cuvette with distilled water. Use this for a blank measurement (Io) at all wavelengths for which the scan is needed. 3. Determine the absorbance A = ln (I/Io) of the Ru(bipy) 32+ at all wavelengths int he range for which the spectrum is required. 4. Plot the absorbance (y-axis) vs. wavelength (x-axis) to obtain the actual spectrum. C. The ionic species tris (2,2’-bipyridine) ruthenium(II) Ru(bipy) 32+ has the structure
and is chiral. Explain briefly how to resolve a racemic mixture of Ru(bipy) 32+ into its two enantiomers, and how to make sure that your separation procedure has worked. 1. Add a chiral reagent (for example l-tartrate) to a solution of the Ru(bipy) 32+ racemic mixture. The tartrate is needed to form diastereomeric salts in the solution. These salts have different physical properties since they are diastereomers. 2. Separate the two salts through differences in their physical properties. In this case, we know that the solubility of the two salts is very different. One of the salts is insoluble and can be filtered out of the mixture, leaving the other salt still dissolved in solution. 3. After recovering and washing the insoluble diastereomeric salt, the pure enantiomer can be recovered by recrystallizing as the dibromide salt. The optical purity of the dibromide salt can be verified by measuring its optical rotation in a solution of known concentration in water. If the solution rotates plane polarized light then at least a partial separation has occurred. Inf no rotation is observed then a racemic mixture is still present. The exact enantiomer recovered can be determined by verifying which direction the plane-polarized light is rotated (using a polarimeter) and comparing this to known experimental results. |