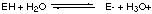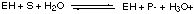Non-NMR Spectroscopies Solutions: #7
7.* (1994 F 9) Visible "spectroscopy". An enzyme EH when placed in water undergoes the reaction
in which equilibrium is rapidly attained and in which equilibrium lies far to the right, i.e. more [E-] than [EH]. A. Write down an expression for the equilibrium constant K for this reaction and show how the concentration ratio [E-] / [EH] depends on pH, i.e. on [H3O+]. K = [E-][H3O+] / [EH] --> [E-] / [EH] = K/ [H3O+]. Increasing pH decreases the [H3O+] (because pH = -log [H3O+] ), thereby increasing [E-] / [EH]. For this particular enzyme the E- form is inactive whereas the form EH rapidly catalyzes the conversion of a substrate molecule S into the products P- and [H3O+], i.e.
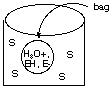
Suppose at time t = 0 a neutral aqueous solution of this enzyme is placed inside a bag that is itself immersed in a neutral solution of the substrate S held in a container:
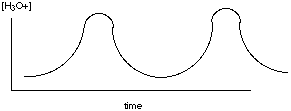
The walls of the bag will not let EH or E- pass through them but are permeable to all other species. Initially, S starts to diffuse into the solution of EH and E- inside the bag. This diffusion process is slow. As S slowly diffuses into the bag, it reacts only with the EH form of the enzyme. At first the reaction rate is small because [EH] is small compared to [E-], but as products are formed (more rapidly than they can diffuse from the bag), [H3O+] increases inside the bag and shifts the rapidly attained enzyme equilibrium to make [EH] increase, which speeds up the reaction with S until a point is reached where S is consumed by reaction more rapidly than it can diffuse into the bag. Following this point P- and H3O+ must diffuse from higher concentration to lower concentration through the bag walls so that the enzyme concentration shifts to the right again lowering [EH] and returning to our starting point. B. Make a rough plot of [H3O+] versus time inside this diffusion-controlled bag (cell) reactor. As the reaction rate happens (first slowly, then more rapidly) [H3O+] increases (first slowly, then more rapidly). Then, the reaction is no longer happening at an appreciable rate and the [H3O+] decreases as it diffuses out of the bag. After enough has diffused out, we’re back to the starting point and the process repeats.
C. Suggest how the [H3O+] concentration variation with time could be visually monitored inside the bag. Add an indicator dye in the bag which absorbs different wavelengths of light when protonated and deprotonated . . . in acidic solution (when the pH is low) it will be protonated (one color) and in basic solution (when the pH is high) it will be deprotonated (another color). Then to monitor the [H3O+] in the bag you could just monitor the color change with time! D. Suppose the product P- has a characteristic UV absorption feature that is different from the UV absorption spectrum of S. Explain how the concentration of P- in the solution inside the container but outside the bag might be monitored in time. Place the container in a UV absorption spectrometer (keep the bag out of the beam though!) and adjust to the wavelength of maximum absorption of the product P-. Alternatively, you could take small samples of the solution outside the bag every 20 seconds or so. The amount of light absorbed is an indication of the concentration of P- as described by Beer’s law. If we know the absorption coefficient of P- at lmax and the path length of the beam through the container, we can determine c = [P-] as a function of time by measuring I / Io over time. |