Non-NMR Spectroscopies Solutions: #5
5. *(1996 2 7) Uv-vis spectroscopy. The following is a schematic diagram of an instrument designed to make absorption measurements: |
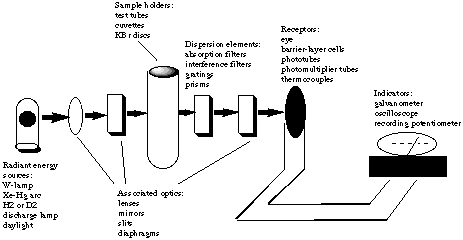
|
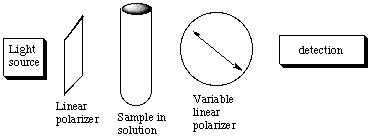
A. The molar absorbance e of Br2 is 368.5 L mol-1 cm-1 at 400 nm. A cell 2 cm long is filled with solution of Br2 of unknown concentration. It is found that 60% of the 400 nm light incident upon it is absorbed. Calculate the concentration of Br2 in this solution. Be sure to state your answer with its units. Recall Beer's Law: I = Ioe(-ecl). In this equation, I is the intensity transmitted through the sample, Io is the intensity of the incident electromagnetic radiation, c is the concentration of the absorbing species, e is the molar absorbance (note: e is dependent on wavelength!), and l is the path length (cell length) over which the radiation is absorbed. In the problem we are given that e = 368.5 L mol-1 cm-1, l = 2 cm, and I/I0 = 0.4, because if 60% is absorbed, then 40% is transmitted. Plugging all of these numbers into the Beer's Law equation, we come up with c = 1.24 x 10-3M. B. A reagent is added to this solution of Br2 that reacts with it to form compounds that do not absorb at 400 nm. As the reaction proceeds, the Br2 is destroyed. Suppose that the rate of Br2 destruction is exponential in time, that is, the concentration c(t) at time t is related to the initial concentration c(0) at time t=0 by c(t) = c(0) exp (-kt) where k is the rate constant. Note that if the time is measured in seconds, then the units of k are inverse seconds. For our solution the absorption of the incident 400 nm light goes from 60% to 20% in 8 seconds after the time the reagent is added to the Br2 solution. Use this information to determine the value of k. Start from Beer's Law: I = Ioe(-ecl) --> ln [I/Io] = -elce(-kt). At t = 0, [I/Io] = 0.4 because if 60% is absorbed then 40% is transmitted; at t = 8 seconds [I/Io] = 0.8 because if 20% is absorbed then 80% is transmitted. Plug in the numbers and divide the two equations for t = 0 and t = 8 seconds to solve for k. ln [0.4] = -elco ln [0.8] = -elcoe(-8k) ______________________________ ln [0.8 / 0.4] = e(-8k) ---> k = 0.1765 sec-1. C. Discuss whether it was necessary to know the values of the absorbance, the path length, or the initial concentration of the Br2 soluction to find the value of k. As shown in part B, the value of k was independent of path length, initial concentration, and molar absorbance, which is quite amazing. Hence time-resolved absorption spectroscopy can be a powerful means of following kinetics (i.e. rathes of changes of concentrations during a reaction). D. Suppose a chiral compound of limited amount has been synthesized. You are asked to determine if the synthesis was successful, that is, whether the compound has a large excess of one enantiomer over the other or is a racemic mixture. Explain how the following setup might be used to accomplish this task. Describe how this device works.
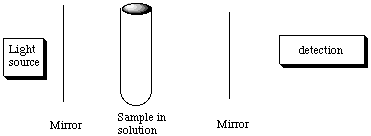
"It takes a hand to recognize a hand." Linear polarized light is the in-phase superposition of left and right circularly polarized light, and the handed molecule interacts with these two kinds of handed light differently, slowing down one more than the other so their phasing is changed, causing the direction of the linearly polarized light to rotate. This is the principle behind circular dichroism (CD) spectroscopy. A reacemic mixture has equal amounts of left-rotating and right-rotating molecules and hence has no net optical activity. If the synthesis gave an excess of one chiral component, the linearly polarized light would be rotated so that the position of maximum to minimum transmission in the variable linear polarizer would change. E. For weakly absorbing samples, it may be advantageous to place the sample between two highly reflecting mirrors. Explain how this device increases the sensitivity of the absorption meausrement.
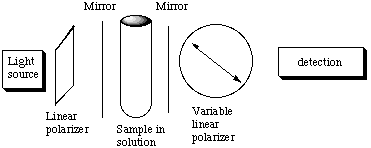
A weak absorption feature means that most of the light will be transmitted. "Highly reflecting" mirrors means that the light will bounce back and forth through the sample many times (on average) before exiting the mirror in front of the detector. Hence the effective path length is greatly extended, often by a factor of 102 to 103. I = Ioe(-ecl). By making l longer, I/Io becomes smaller on a weak absorption feature and hence more detectable. In other words, since l is larger, c can be smaller so you can detect absorption more sensitively. F. It might seem natural to combine these two setups:
Actually, this combination is a poor idea. Explain why the above setup is essentially without merit for increasing the sensitivity for measureing optical activity. Suppose the plane of the linearly polarized light is rotated by x degrees clockwise as seen from the light source as the light propagates from left to right toward the detector. The right mirror bounces the light back toward the left mirror and again the plane of polarization is rotated x degrees clockwise as seen from the righthand mirror. But this is counterclockwise as seen from the lightsource (-x degrees). So by the time the light makes it back to the left mirror there is no net rotation. The light that is detected has the same net roation as it it made no reflection at all. Meanwhile, the light is losing intensity as it bounces back and forth (see part E). So we get no increase at all in the sensitivity for measuring optical activity. |