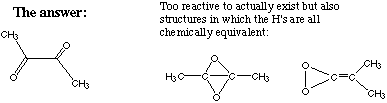Non-NMR Spectroscopies Solutions: #3
3.* (1997 2 5) Mass spectroscopy A. An unknown pure compound X has a mass spectrum in which the most prominent peak occurs at m/z = 86. Assume that X contains C and H but that O may or may not be present. Write down all possible molecular formulas for X that make chemical sense.
B. Closer inspection shows that in addition to the ion at m/z = 86 there is a weaker mass peak at m/z = 87. The intensity ratio of the weaker to the stronger is 4.7% plus or minus 0.5 %, where the uncertainty represents a 99% confidence level. What can you conclude about the molecular formula for compound X? The peak at m/z = 87 is probably due to the occurence of one atom of 13C in the compound. 13C occurs instead of 12C one ninetieth of the time. The expected intensity ratios for each of the four preceeding possible compounds are as follows: C6H14: I87I86 = [6*(89/90) 5 (1/90) 1] / [(89/90) 6] = 6/89 = 0.067 C5H10O: I87I86 = [5*(89/90) 4 (1/90) 1] / [(89/90) 5] = 5/89 = 0.056 C4H6O2: I87I86 = [4*(89/90) 3 (1/90) 1] / [(89/90) 4] = 4/89 = 0.045 C3H2O3: I87I86 = [3*(89/90) 2 (1/90) 1] / [(89/90) 3] = 3/89 = 0.033 Therefore, we can say with 99% confidence that X is C4H6O2. C. 1H NMR of compound X shows one peak. What are the chemically sensible structures for compound X? One NMR peak implies that all the H atoms are magnetically equivalent.
D. Further inspection of the mass spectrum for compound X shows a prominent peak at m/z = 71 and m/z = 43. Explain how these peaks come about. m/z = 71 comes about through loss of a methyl group during the bombardment. m/z = 43 comes about through loss of a methyl and a C=O or through double ionization of the parent molecule.
|