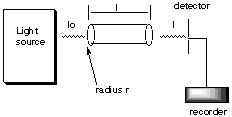Non-NMR Spectroscopies Solutions: #2
2. * (1997 F 16) Beer’s Law and kinetics. (NOTE: This problem contains both spectroscopy and kinetics. Before understanding kinetics, it’s not going to make much sense.) A cylindrical tube of length l and radius r is placed between a light source of wavelength l and a light detector.
The tube is filled with water and the transmitted intensity is found to be Io. Then x moles of A are added to the water in the tube. Compound A completely dissovles in water and undergoes a bimolecular reaction to form the dimer A2 which absorbs light at wavelength l with an extinction coefficient e. Compound A is transparent at wavelength l. The rate constant for the dimerization step is kd. Derive an expression for how the transmitted intensity at wavelength l changes with time, i.e. find an expression for I(t). Beer’s Law: I = Ioe(-ecl), where c depends on t according to c = [A2(t)]. We know that vol = pr2l and [Ao] = moles / volume = x / pr2l. Because we are told that this is a bimolecular reaction (i.e. two molecules of A must collide to form the dimer, we know that -d[A] / dt = kd [A]2. Doing the rate law integration, we come up with 1/[A] - 1/[Ao] - kdt --> 1/[A] = kdt + 1/[Ao] = ([Ao]kdt + 1)/[Ao] --> [A] = [Ao] / (kdt[Ao] + 1). We also know that [A] + 2[A2] = [Ao] at any point during the reaction, because A can’t just spontaneously dissappear. Thus, [A2] = ([Ao] - [A]) / 2. Now all we have left to do is to plug into the immediately preceeding equation our derived [A] from above. Thus we obtain: [A2(t)] = ([Ao] - [A]) / 2 = ([Ao] - {[Ao] / (kdt[Ao] + 1)}) / 2 = kdt[Ao]2 / 2kdt[Ao] + 2). Now plug this whole big ugly thing into the very first expression for I above. Final answer: I(t) = Io exp [-elkdt[Ao]2 / 2kdt[Ao] + 2)], where [Ao] = x / pr2l.
|