Non-NMR Spectroscopies Solutions: #1
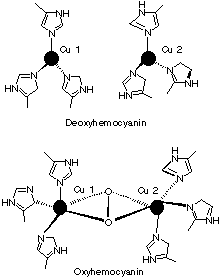
1.* (1997 F 9) Identifying the useful spectroscopy. Hemocyanins, non-heme bimetallic copper proteins, are oxygen carriers in anthropods such as spiny lobster. Below the core structures of deoxy- and oxyhemocyanin are displayed. The protein which provides the histidine ligands is not shown. Deoxyhemocyanin has both copper ions in the +1 oxidation state. Oxy-hemocyanin is diamagnetic.
A. Indicate the oxidation state of the copper ions and of the O2 ligand in oxyhemocyanin. We are told that de-oxy hemocyanin has both coppers in the +1 oxidation state, and we know that oxygen typically oxidizes metals. Therefore we conclude that both of the coppers are +2; both of the oxygens are -2. B. What single method: mass spectrometry, UV-visible spectroscopy, NMR, microwave, or infrared/Raman spectroscopy, would be best suited to verify the oxidation state of the O2 ligand in oxyhemocyanin? Briefly explain your answer--for example, would oxgen isotopes such as 18O2 be useful? IR/Raman of the O2 stretch would verify the oxidation state of O2 because the stretching frequency of O2 is characteristic of its oxidation state. With 18O2 the frequency shift (isotope effect) can be used to confirm the assignment of the IR/Raman band. C. Indicate the number of electrons in the copper ions within both deoxy and oxy hemocyanin. d10 for Cu(I) deoxy d9 for each Cu(II) in the oxy D. Why do you think oxyhemocyanin is diamagnetic? The 2 unpaired electrons, one on each Cu+2, couple by a bonding interaction through the bridging peroxide ligand. |