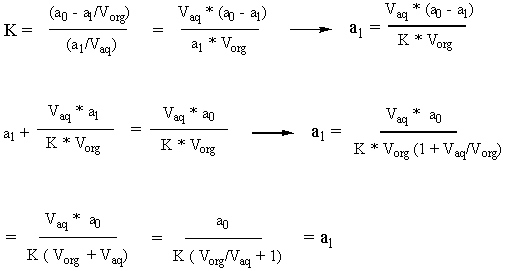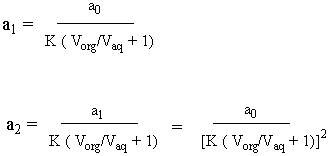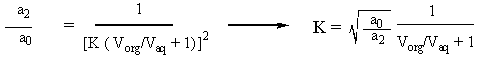Separation Technologies Solutions: #3
3.* (1996 2 8) What is the value of the minimum partition coefficient K, defined as K = [A]org/[A]aq that would permit removal of 99% of a solute A from 50.0 mL of water with two 20.0 mL extractions with toluene? Note the separation "reaction" A(aq)<------>A(org) governed by the equilibrium constant . The partition coefficient: K = [A]org/[A]aq. Here toluene (methylbenzene) is the organic solvent. Let
Let
We start from the definitions of K and molarity (moles/volume). Our goal is to solve for a2/a0 so that we can set it equal to 0.01, meaning that 99% of a0 is gone. But before we can get a2 in terms of a0, we have to get a1 in terms of a0. Happily, we discover that a0 is related to a1 by a simple factor.
This tells us that the number of moles in the in the aqueous phase after an abstraction is proportional to the number of moles in the aqueous phase before that abstraction by the proportionality constant shown above. So if we do two abstractions, the following ensues:
Therefore:
Since we want 99% of the material abstracted, we know that a2/a0 = 0.01. Additionally, we are given numbers for Vorg and Vaq in the statement of the problem. So all we have to do is plug in the numbers and solve for K, which turns out to be 22.5. |