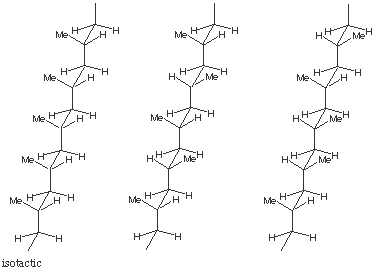
Polymers Solutions: #5
5.* (1996 3 3) Write in turn the stereochemical structures of isotactic, syndiotactic, and atactic polypropylene. Indicate which form is the most stable in solution (has the lowest free energy) and which would have the lowest melting point. Briefly explain your answer.
The atactic extreme will have the lowest melting point and highest DG of solvation. |