Polymers Solutions: #3
3.* (1996 3 1) Discuss the structural features of a polymer which would make it a useful elastomer; specifically describe the underlying thermodynamic property which causes a stretched elastomer to return to its unstretched state. Mention the effect of molecular stereoisomers. Discuss the role of inter (between) chain interactions and mention those factors which determine the temperature range over which the elastomer is useful–for example what happens on a molecular level at low temperatures. Illustrate your discussion with an actual example, using molecular formulas. •What makes a useful elastomer: A useful elastomer has enough crosslinking (via vulcanization, hydrogen bonding, dipole-dipole interaction,etc.) between polymer chains to provide a restoring force, but not so much crosslinking that it is crystalline. A useful elastomer also has a low Tg (glass transition temperature: the temperature below which it goes into the glass state and becomes brittle). •Thermodynamics: The underlying thermodynamic property which provides the restoring force is entropy DS. DS > 0 if you go from stretched to unstretched because there are more conformations available in the relaxes state, so there is more disorder. •Stereoisomerism: •stereoregular/isotactic: higher intermolecular interactions, better packing, more crystalline. •stereoirregular/atactic: lower intermolecular interactions, less crystalline, more "gooey" and inefficiently packed. •double bonds: cis bonds tend to make for less efficient packing forces. The presence of such bonds cause the polymer to be less crystalline, more rubbery. On the other hand, trans bonds enforce a more rodlike structure. •Interchain forces: The stronger the forces (covalent crosslinks, hydrogen bonds, dipole-dipole interactions, etc) the more crystalline and rigid. Polymers with very little interchain interaction are viscous and gooey. •Temperature: Polymers have a glass transition temperature Tg, below which the polymer is hard and crystalline. Above Tg, thermal energy overcomes some intermolecular forces and the polymer is either elastomeric or gooey. Some polymers are so rigid that heating leads to decomposition before approaching Tg. Useful Elastomers: •Vulcanized rubber is a great elastomer. 0% vulcanized is gooey, 2% vulcanized is elastomeric, and 5% vulcanized is hard. 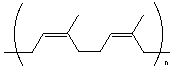
•Non-useful elastomers: Nylon has too many hydrogen bonds, so its interchain forces are too strong. Any polymer with a lot of crosslinking fails to be a good elastomer. |