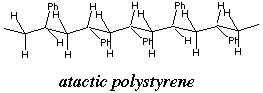Polymers Solutions: #2
2.* (1997 3 1) Compounds like styrene, PhCH=CH2, are unstable as pure liquids. Such olefins tend to explode, even in the absence of oxygen. Particles of a sticky solid, which is soluble in benzene, can be found in debris resulting from the explosion. A. Explain the origin of the explosive decomposition of styrene and of the heat which is given off. You may find it useful to refer to the table of bond energies below.
The olefin polymerization breaks a pi bond (the energy of which is 145.8 - 82.6 Kcal/mol) and forms a single bond (the energy of which is 82.6 Kcal/mol) for each reacting mol of styrene. The net enthalpy change of this reaction is computed by subtracting the enthalpy of bonds formed from the enthalpy of bonds destroyed, because you have to put in energy to break bonds and energy is released when bonds form. Thus DH = (145.8 - 82.6 - 82.6)Kcal/mol = 19.4 Kcal/mol, which means that the reaction is highly exothermic; hence the explosion. B. Write a plausible structure of the sticky solid which results from styrene decomposition. Very briefly describe the structural possibilities. Structure: (-CH2-CHPh-CH2-CHPh-CH2-CHPh-) Its stickiness is your clue that it’s atactic.
Structural possibilities: atactic, syndiotactic, isotactic |