Polymers Solutions: #14
14.* (1991 2 6) Consider a polymer X with empirical formula (CH)n. It is a gummy polymer that becomes a more brittle polymer (Y) when hydrogenated. Deduce the structure of X and Y from these data. X's empirical formula leads us to conclude that each carbon is bonded to one H and 2 other carbons. Because X can be hydrogenated, it must have double bonds. Each carbon in X, therefore, has one C=C bond, one C-C bond, and one C-H bond.
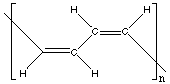
X can have double bonds that are cis or trans. Because X is gummy, it can be deduced that it's the cis isomer, which is likely to form coils as opposed to the rigid rods formed by the trans isomer. Y is the hydrogenated form of X. Each carbon is bonded to 2 hydrogens and two carbons. 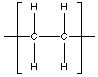
|