Polymers Solutions: #11
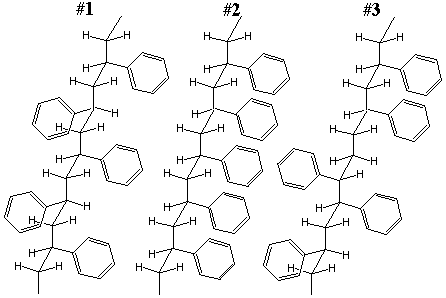
11.* (1993 2 2) Below are structures for stereoisomers of polystyrene.
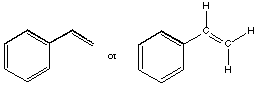
A. Draw a structure for the monomer from which polystyrene is prepared.
B. Write down the tacticity of each of the numbered polymers: #1 is syndiotactic, #2 is isotactic, and #3 is actactic C. Which structure do you expect to exhibit the highest melting point and which would have the lowest melting point? The isotactic polymer will have the highest melting point due to its packing efficiency and high interchain forces. D. What physical methods might you use to distinguish these stereoisomers? Suggest two and explain. X-ray crystallography can be used to elucidate the structures and look for different degrees of crystallinity. 1H or 13C NMR can be used to assign tacticity. Checking the melting point and density will verify which one is isotactic; the isotactic one will have both the highest mp and the highest density. E. Under what conditions does polystyrene exhibit elastic properties? Why is polystyrene not used as an elastomer? Polystyrene exhibits elastic properties above its glass transition temperature (Tg is about 100oC.) It is not used as an elastomer because its glass transition temperature is too high above room temperature to be practical. |