Polymers Solutions: #10
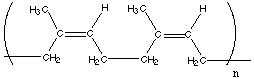
10.* (1994 2 3B) When natural rubber, polyisoprene (shown below) is treated with a small amount of elemental sulfur, the resulting product ("vulcanized rubber") is a useful elastomer; before treatment with sulfur, polyisoprene is a gooey material. Upon stretching, such unvulcanized polyisoprene flows and exhibits little restoring force; it is not a useful elastomer. In general terms, explain what the sulfur is doing to make vulcanized rubber useful.
Natural rubber (cis-polyisoprene) before vulcanizing with sulfur. Sulfur reacts with some of the olefinic (C=C) bonds in the polymeric chains and forms sulfide and/or disulfied bridges between polyisoprene chains. These bridges or chemical crosslinks prevent the polymer from flowing when stretched. The polymer thuys becomes a useful elastomer. C. Professor Robert Waymouth (Stanford Chemistry) recently invented and patented a useful elastomer (a rubber) made entirely from propylene (1-propene). This material, which should compete with the elastomer used in "Air Jordan" basketball shoes, has no olefinic bonds, does not react with sulfur but nevertheless is a useful rubber; Waymouth’s new elastomer exhibits a strong restoring force when stretched. Waymouth’s compound is a block copolymer comprised of alternative sections of isotactic and atactic polypropylene. Pure isotactic polypropylene is not useful as an elastomer; it is high melting and is employed as a fiber for carpets, shirts, etc. Pure isotactic polypropylene does not stretch very much before breaking at room temperature. By contrast, pure atactic polypropylene is lower melting; when stretched this polymer flows. Atactic polypropylene shows little restoring force upon stretching, it flows–much like unvulcanized polyisoprene (or well chewed chewing gum). In two or three sentences, propose a molecular explanation for the useful elastomeric properties of Waymouth’s new block copolymer. Isotactic polypropylene forms coils. These coils pack in a very ordered manner and thus have large crystalline regions. Atactic polypropylene, on the other hand, forms a highly amorphous material with only weak interactions between chains. In the block copolymer, there are regions which have some crystallinity and thus form physical crosslinks between chains, and there are other regions which are mostly amorphous. The combination of highly amorphous (and thus randomly coiled) regions and crystalline regions provides the correct properties to form an excellent elastomer. D. Why can't one use sulfur to vulcanize atactic polypropylene? Polypropylene does not have any C=C bonds. Thus, elemental sulfur cannot react to form crosslinks between polymer chains. |