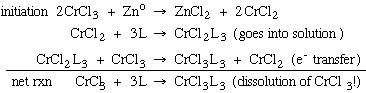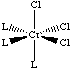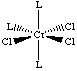Organometallic Solutions: #8
8.* (1994 F 6) Anhydrous chromium trichloride (CrCl3) is insoluble; in the solid state CrCl3 exists as a three-dimentsional polymeric bridging chloride structure. By contrast, the hydrate, CrCl3(H2O)3 is soluble in water; it does not exchange with radioactive chloride anions over a period of many days in solution. Chromium dichloride (CrCl2) is also soluble in H2O; the chloride ligands in CrCl2 are rapidly exchanged by H2O forming Cr(H2O) x2+. A. Propose a mechanism for this process by writing equations for the steps which may occur. (Hint: Eo for Zn2+/Zn- is -0.7 V vs. NHE.) Zn metal is used to produce a trace amount of Cr2+ in solution which then catalytically solubilizes CrCl3 through electron transfer (Cr(III) is inert to substitution, Cr(II) is labile).
B. Give a rationale for the difference in the rates of chloride exchange comparing Cr(III) and Cr(II).
C. Discuss the number and nature of isomers formed by CrCl3(L)3. Draw structural formulas depicting these stereoisomers.
D. How would you separate such stereoisomers? Since they are diastereomers, they can be separated via standard techniques (physical / chemical differences): crystallization, chromatography… |