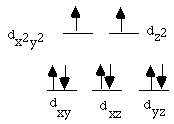Organometallic Solutions: #7
7.* (1995 2 3) Paramagnetic complexes of Pd are quite rare whereas Ni complexes are often paramagnetic. It is also true that octahedral complexes of Pd(II) are virtually unknown whereas four-coordinate complexes of Pd(II) are common. A. What is the electron count in 4-coordinate Pd(II) complexes? Are these complexes "coordinately" saturated? These complexes are d8. They have 8 + 4x2=16 d electrons, and are unsaturated. B. What is the typical coordination geometry manifest by four-coordinate Pd(II) complexes? The typical coordination geometry found in four-coordinate Pd(II) complexes is square planar. C. Propose a d orbital splitting pattern for an octahedral complex; mark each orbital by the usual Cartesian coordinates X, Y, Z assuming that the ligands lie on the X, Y, and Z axes. Show patterns of orbital degeneracies. Fill in the correct number of electrons derived from the oxidation state Pd(II). How many electrons are unpaired? Orbital splitting pattern is shown below:
There are 2 unpaired electrons. D. What accounts for the difference between the diamagnetism of Pd(II) complexes and the paramagnetism of Ni(II) complexes? Pd(II) is a 4 d transition metal; thus it is low spin and the energy gap between levels is large. In the square planar geometry, it is possible for the Pd(II) complex to leave the highest energy (most antibonding) orbital unoccupied with all other levels completely filled. This accounts for its diamagnetism. Ni(II), on the other hand, is a 3d transition metal, so for some ligands it can be high-spin. Therefore there is a high probability that an Ni(II) complex will be paramagnetic. |