Organometallic Solutions: #4
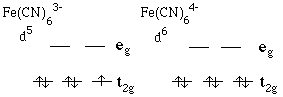
4.* (1995 3 1) One can make "educated guesses" about the rates of outer sphere electron transfer reactions. For each of the following suggest whether the "self-exchange" is either very fast or not very fast. Briefly indicate the basis for your guess. A. Reaction between molecular oxygen, O2, and the superoxide ion, O2-NOT VERY FAST: O2 has 2 unnpaired electrons in the p* orbital. Self exchange will put another electron in the antibonding orbital, thus resulting in change of bond length. Bond order of O2 = 2, bond order of O2- = 1.5. B. Fe(CN)63- and Fe(CN)64- (do not worry about like charges repelling on another). very fast exchange
Transfer of electrons takes place between non-bonding t2g orbital, thus no change in structure or bond length. |